Occupational Health and Safety Act, 1993 (Act No. 85 of 1993)RegulationsRegulations for Hazardous Biological Agents, 2022AnnexuresAnnexure B: Biohazard Sign |
ANNEXURE B
[Regulations 10(2)(f), 11(4)(b) and 14(b)]
BIOHAZARD SIGN
Explanatory notes to the Regulations for Hazardous Biological Agents
The purpose of this document is to provide guidance to all employers and employees who are responsible for or concerned with the control and prevention of hazardous biological agent risks in the workplace.
This guide does not replace the Hazardous Biological Agents Regulations of 2021. It is intended to give practical insight into the applications of the Regulations. It should always be read in conjunction with the HBA regulations and the Occupational Health and Safety Act of 1993.
Wearing and use of protective clothing and respiratory protective equipment.
| 1. | Where the HBA exposure cannot be prevented by other means, individual protection measures including PPE must be used. Workers have to be provided with appropriate protective clothing or other appropriate special clothing (ref: Directive 2000/54/EC of 18 September 2000 of the European Parliament on the protection of workers from risks related to exposure to biological agents at work). According to standard EN 14126, protective clothing against biological hazards is classified in accordance with leak tightness and is recognised by the suffix B, e.g. type 3-B. |
| 2. | In the selection of the protective clothing, one should note that the efficacy offered by the protection. The larger the number of the protection class, the better is the clothing for that specific property. |
| 3. | The user has to be able to perform all the movements, assume the working positions he or she will have when performing the work, and be able to use the working tools. In order to ease the work load, the clothing should be selected so that its donning and removal are easy. The removal has to be straightforward also since different kind of emergencies may arise, and the clothing may need to be taken off quickly. A poor fit of the clothing may result in reduced efficacy of the clothing. |
| 4. | If other PPE are needed together with the protective clothing, the efficacy of the entire PPE has to be ensured. Special care has to be taken to ensure that the wearer, who has to wear hearing protection will be protected and be able to communicate and hear warning signals. Wearer trials are needed to ensure the usability of the protective clothing. An evaluation of the maintainability of the clothing is also needed before the selection. The purchase of protective clothing should always be based on a risk assessment. |
| 5. | User training must include donning and removal of the protective clothing. Also, pre-use checks (e.g.checking for defects in ensemble assembly, garment and components, accessory, interface (closure, zippers), sufficiency of ventilation rate (gas-tight clothing)), safe work methods and monitoring the clothing while in use. The training should be carried out under realistic conditions and with actual equipment following the same procedures as in the real work task. In user training, the final check of size, fit, and compatibility must be investigated. |
| 6. | Decontamination permits the reuse of the types of protective clothing and equipment that are reusable. It can be made through physical or chemical methods to inactivate the contaminant or by using combination of these techniques. The decontamination procedure should not put other people or the environment at risk or damage the PPE. The effectiveness of decontamination should be checked e.g. visually searching for signs of discolorations, swelling, corrosive effects, stiffness or degradation of the material. Single use clothing is used when the contamination cannot be effectively removed from the clothing. Single use clothing is commonly used against microbiological agents. |
| 7. | The storage must be arranged to prevent damage to the protective clothing and equipment. Exposure to sunlight, dust, moisture, chemicals, extreme temperatures and mechanical damages e.g. folding must be prevented. Potentially contaminated protective clothing and equipment must be stored separately from unused protective clothing. |
| 8. | Regular inspection is necessary and should include inspection when the protective clothing and equipment is first received, inspection when it is selected for a particular task, inspected after use and previous maintenance. Records must be kept of all inspection procedures containing item identification number, date of inspection, person conducting the inspection, results, and unusual findings. |
| 9. | In all repair work, the manufacturer's instruction must be followed or the personal protective clothing and equipment must be sent to repair location authorised by the manufacturer. |
The emergency preparedness plan
| 1. | The development of an emergency preparedness plan should be based on all hazards and assessments of risks, and of the available capacity to manage the priority risks. The objective of an emergency response plan is to provide practical ways to reduce the risk of employee's exposure to the disease in the workplace and to deal with any unforeseen situations. The plan should outline actions that employers and employees must take in the event of an emergency situation to ensure their health and safety. The plan should be communicated to all employees, contractors and suppliers. Everyone must be aware of what they should do - or not do - based on the plan, including their duties and responsibilities. |
| 2. | The plan must clearly outline the procedures to be followed in the event of an emergency. Such procedures should include: |
| • | risk assessment; |
| • | ways to alert employees; |
| • | Evacuation; |
| • | emergency response; |
| • | designated assembly locations; |
| • | contact people and their telephone numbers; |
| • | first aid and medical assistance; |
| • | clean-up and business resumption; |
| • | reporting emergencies (reporting exposures, incidents, accidental release); |
| • | employee training; |
| • | exposure control procedures (engineering controls, employee training and workplace practices, personal protective equipment) |
| • | ways of testing the plan (drills). |
Duties of persons who might be exposed to HBA
| 1. | In addition to the duties indicated in the regulation 5, employees' must report any deviations in the adherence to control measures put in place by the employer to mitigate exposure of a medical nature. This allows employees exposed to HBA to take responsibility to inform the employer of any challenges experienced with control measures put in place as opposed to employee not adhering to measures or being subjected to ill effects of measures without the employer's knowledge. |
Competent person
| 1. | Is a person who has,in respect of the work or task to be performed, the required knowledge, training and experience and, where applicable, qualifications specific to hazardous biological agents: Provided that, where appropriate qualifications and training are registered in terms of the National Qualifications Framework Act, 2008 (Act No. 67 of 2008), Skills Development Act,(Act No 97 of 1998) Chapter 6C as well as the Continuing Education and Training Act 16 of 2006, those qualifications and that training must be regarded as the required qualifications and training; and |
| 2. | is familiar with the Act and the applicable regulations made under the Act; |
| 3. | In general, for people to be competent in the health and safety aspects of their work, they will have a combination of the following requirements: |
| • | be qualified because of knowledge, training, and experience to do the assigned work; |
| • | have knowledge about the hazards and risks associated with the job or task to be performed (e.g., knows what hazards and risks are present); |
| • | know how to recognize, evaluate and control these hazards and risks (e.g., knows what precautions to take or controls to use/are in place for the different hazards or risks); |
| • | have the ability to work so that their health and safety and the health and safety of others is not in danger; |
| • | have knowledge of the laws and regulations that apply to the work being done. |
| 4. | The level of competence required will depend on the complexity of the situation and the task involved. |
| 5. | In all cases, it is the employer who should be able to justify the basis on which a worker is considered to be "adequately qualified", "suitably trained" or "sufficient experience". It is not possible to provide a general list of the exact knowledge, training and experience required. Every organisation must determine the requirements for each position or task to be done. Frequency of competency assessment will depend on the trends of risk outcomes (e.g. incidents). changes in technology or contraventions by the inspectorate which may indicate a need to increase the level of competency. |
Confidentiality in relation to records
| 1. | Confidentiality is the right of an individual to have personal, identifiable medical information kept private. |
| 2. | Health records are different to medical records in that they should not contain confidential medical information. Health records and medical records must therefore be kept separate to avoid any breaches of medical confidentiality. Any personal medical information should be kept in confidence and held by the occupational health professional responsible for the health surveillance programme. The doctor or nurse should only provide employers with information on fitness to work and any restrictions that may apply in that respect. |
| 3. | Medical records can only be released to third parties, such as the employer, in accordance with the Protection of Personal Information (POPI) act and constitution is also applicable. |
Biological containment and medical fitness restrictions
| 1. | Biocontainment is a component of biorisk management. The overall objective of biological containment is to confine a hazardous biological agent, thereby reducing the potential for exposure to workers or other persons, and the likelihood of accidental release to the environment. |
| 2. | A medical fitness certificate is a document completed by a qualified occupational health practitioner or an occupational medical practitioner. The employee fitness certificate is to ensure that the employee is fit for the task or job he or she is to perform according to his job specification |
Safety equipment (Primary Barriers and Personal Protective Equipment)
| 1. | The primary means of physical containment include the use of containment equipment including safety equipment includes biosafety cabinets (BSCs), personal protective equipment (PPE), enclosed containers,and other controls designed to remove or minimise exposures to hazardous biological materials. |
| 2. | Personal protective equipment is specialised clothing or equipment worn by workers to provide another layer of protection while handling hazardous biological agents. PPE may include respirators, gloves, safety glasses, lab coats or gowns, and other protective clothing. Biosafety Cabinets are primary containment devices designed to contain hazardous biological agents. |
Facility Design and Construction (Secondary Barriers)
| 1. | The facility design and physical features should provide primary barrier protection from the accidental release of hazardous biological agents outside the facility or to the environment. The design and construction of the facility contribute to the laboratory workers' protection. It also provides a barrier to protect people, animals, and the environment outside of the facility from hazardous biological agents that may be accidentally released from the facility. Small and large animal laboratories require additional design considerations to allow for feeding, housing, handling, and containment. These facilities are defined by Animal Biosafety Levels (ABSL) or Biosafety Level-Agriculture (BSL-Ag). |
| 2. | The use of specific containment equipment and procedures is determined through risk assessments conducted at individual institutions. Important differences exist between risk assessment criteria for public health and worker protection, and requirements for animal, wildlife, plant, and agricultural containment. |
Control measures related to appropriate disinfection
Disinfectants must be appropriate for the relevant biological agents or hazards identified and must be used in accordance with the manufacturer's instructions to ensure adequate contact time. Always refer to the safety data sheets to ensure safe use of the product. The following documents will provide further information:
| 1. | Practical Manual for Implementation of the National Infection Prevention and Control Strategic Framework, NDOH, March 2020. |
| 2. | COVID-19 Disease: Infection Prevention and Control Guidelines, NDOH, April 2020 |
Fit testing of Personal Protective Equipment
| 1. | To ensure that a respirator is effective at reducing risk, it is important to conduct respirator fit testing in order to match the user according to their facial characteristics with the correct size and style of the respirator, especially for those working in high risk environments. Respirator fit testing can be either qualitative or quantitative and it is an important element of a respiratory protection programme. Fit testing forms a key part of achieving the objective filtration of hazardous biological agents in protecting the user. |
| 2. | Quantitative fit testing is defined in ANSI Z88.2-1992 as "A fit test that uses an instrument to measure the challenge agent inside and outside the respirator." This procedure is more precise than the qualitative fit test. Qualitative fit testing is defined in ANSI Z88.2-1992 as "a pass/fail test that relies on the subject's response to detect the challenge agent.' Since this test relies on the subjective response of the user,the reproducibility and accuracy may vary. |
| 3. | Fit testing should be performed at least once annually for workers who is required to wear a particular respirator per specific respirator brand and size. It is also recommended immediately if the user experiences a weight change of 10kg or more, has significant dental changes, or has reconstructive surgery or a facial disfigurement (scarring). |
| 4. | Fit testing should not be confused with a respirator fit check. ANSI Z88.2-1992 defines a fit check as "a test by the user to determine if the respirator is properly sealed to the face." It is recommended that a fit check be performed each time the respirator is donned or adjusted. The fit check is a quick method to determine if the respirator is properly sealed to the face. Under part A.6 of ANSI Z88.2-1992, procedures for conducting a fit check are described. The two most commonly performed methods are the positive and negative pressure tests. |
| 5. | The positive pressure check requires the user to cover the exhalation valve (if present in the case of elastomeric filtered respirators suggested in times of extremely constrained supply) of the tight-fitting respirator (placing the palm over the valve is usually sufficient) and exhale. If there is no indication of air escaping, the fit is considered satisfactory. The wearer then inhales. If no leakage is detected,the face piece seal is satisfactory. |
| 6. | For valved masks during a negative pressure fit check, the inlet opening of the respirator's cartridges or filters are covered prior to inhalation. Fit checking requires exposing the wearer to a challenge agent (isoamyl acetate, saccharin mist, irritant fume). If the wearer does not detect the challenge agent, the fit check is successful. This method is the only way respirators without valves can be effectively tested. |
Reference: NDOH. Policy for the regulation of quality respiratory protective equipment (RPE) supply in healthcare. 2020.
Transport of HBA
In addition to the legislation mentioned in the regulations, the employer shall ensure that transport of biological materials internally or externally is in accordance with the organization's risk assessments. The employer shall address all applicable international, national and local transportation requirements and ensure that a system is in place to maintain appropriate controls on shipping packages and transport containers that contain biological materials in accordance with the organization's risk assessments.
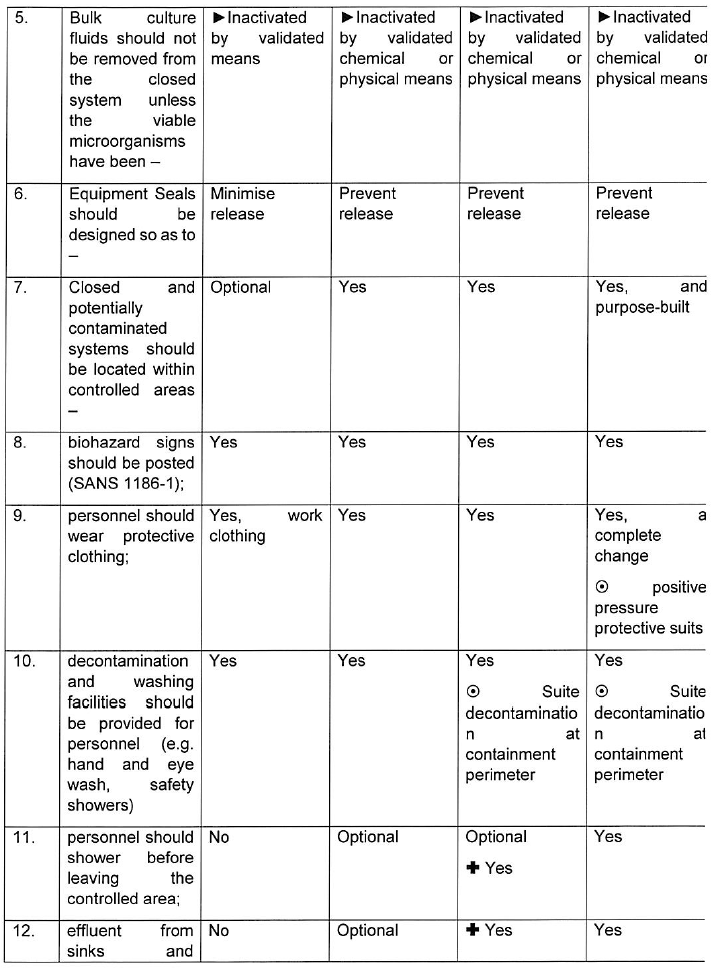
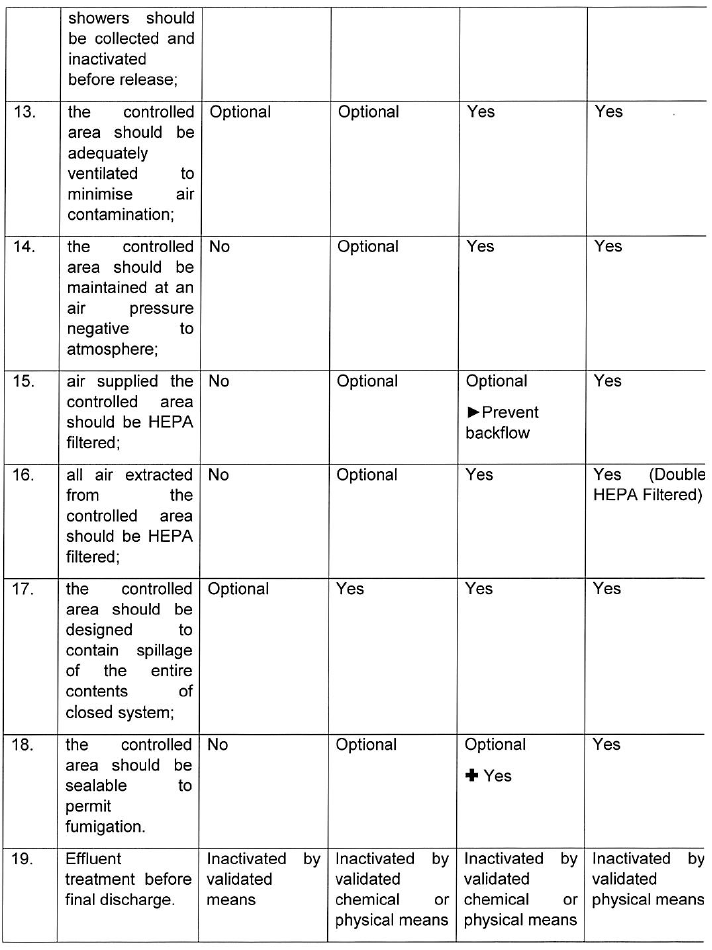
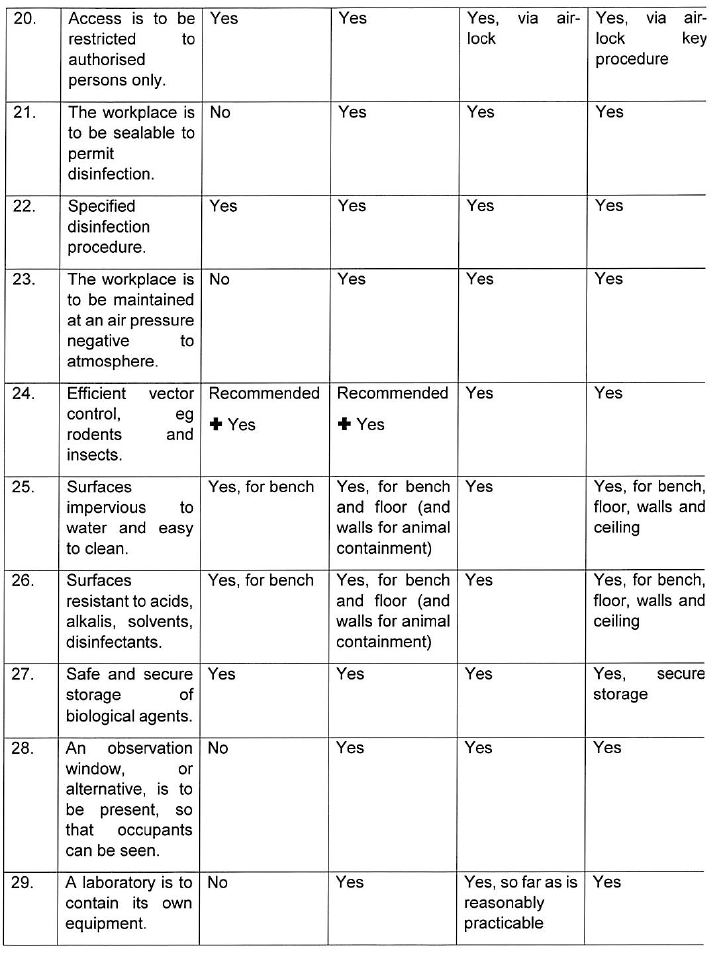
INDICATIONS CONCERNING CONTAINMENT MEASURES AND CONTAINMENT LEVELS
For group 1 biological agents, including life-attenuated vaccines, no physical containment measures are prescribed below. For work with group 1 biological agents the principles of good occupational safety and hygiene should be observed.
Where hazardous biological agents can be transmitted through suspended aerosols over long distances they are classified as airborne spread in the table below. Mechanism of transmission including contact, droplet and vector spread are considered as non-airborne spread below.